Help
This page contains a number of frequently asked questions and answers.
Answer
The European Rare Blood Disorders Platform (ENROL), has been conceived in the core of the European Reference Network on Rare Hematological Diseases (ERN-EuroBloodNet) as the umbrella platform for both new and already existing registries on Rare Hematological Diseases (RHD).
ENROL aims is to facilitate the best health planning and to promote basic, translational and clinical research in RHD by enabling epidemiological surveillance and identification of trial cohorts at the EU level. For this, ENROL will obtain comparable EU data for RHD on demographics, survival rates, diagnosis methods, genetic information, main clinical manifestations and treatments by promoting the interoperability standards for patient registries in line with the European Platform on Rare Disease Registration (EU RD Platform). ENROL is officially endorsed by the European Hematology Association (EHA).
Answer
The overall governance of ENROL is undertaken by a Consortium formed by the Institutions of the Principal Investigators (PIs) of the platform:
-
Vall d’hebron Research Institute - Vall d’Hebron Research Institute - University Hospital Vall d’Hebrón (VHIR/HUVH), Barcelona, Spain.
PI: Dr Maria del Mar Mañú Pereira -
Hôpital ERASME (ERASME), Brussels, Belgium.
PI: Prof Béatrice Gulbis -
Cyprus Institute of Neurology and Genetics (CING), Nicosia, Cyprus.
PI:Dr Marina Kleanthous -
Assistance Publique - Hopitaux de Paris (AP-HP), France.
PI: Pierre Fenaux
ENROL is co-funded by the Health Programme of the European Union (under the call for proposals HP-PJ-2019 on Rare disease registries for European Reference Networks GA number 947670).
Answer
ENROL disease coverage results in more than 450 different rare hematological diseases entities with differential clinical and aetiological features i.e. oncological vs non-oncological, hereditary vs acquired, or significant difference frequency, which can be classified into the following disease groups:
- RAD: Inherited Rare Anaemia Disorders, including inherited Bone Marrow Failures
- BMF: Acquired Bone Marrow Failures
- Bleeding: Rare bleeding-coagulation disorders and related diseases
- HH-Iron: Hemochromatosis and other rare genetic disorders of iron metabolism and heme synthesis
- Myeloid: Myeloid malignancies
- Lymphoid: Lymphoid malignancies
ENROL dataset has been conceived in a bottom-up design for ensuring the capture of the common elements for rare diseases in line with the EU recommendations and with the EU RD platform, and the key features common for the whole spectrum for RHDs. The latest level can be stepped up for the definition of domain specific elements that support the in-depth analysis.
In the spirit of ENROL aims to promote the connection and linkage of available data sources and the creation of new registries where lacking, ENROL has established collaborations for supporting the upgrade/creation of two pilot European registries for the domain specific data gathering in compliance with the standards of interoperability and ENROL policy focussed on:
- Rare Anaemia Disorders (RAD)
- Blastic plasmacytoid dendritic cell neoplasm (BPDCN)
Answer
The ENROL strategy for data gathering combines the exhaustiveness of data collection at EU level for health planning and epidemiological porpoises, with a higher level of RHD data granularity for promoting research and identification of patients’ cohorts.
The platform has been designed to integrate data from available sources namely a) healthcare providers hospital records, and b) EU/national/local existing registries, with different levels of granularity to pursue ENROL aims:
- Counts/aggregated level to facilitate the increase of exhaustiveness of data gathered at the EU level required for the epidemiological surveillance,
- Pseudonymised individual data to promote research and facilitate the identification of trial groups.
As final result, ENROL will not only provide epidemiological surveillance for RHD, but also facilitate the re-use of data by exploratory study population queries aiming to identify adequate target populations to include in clinical trials and research projects. This will promote the development of clinical trials, especially in those countries with limited access to drugs, and –OMICS based research on drug-able targets.
Answer
ENROL is built in compliance with the European Union’s General Data Protection Regulation (EU) 2016/679, on the protection of natural persons with regard to the processing of personal data and on the free movement of such data.
Personal data allowing patients' direct identification and contact will not be collected by ENROL. Instead, medical information will be collected by ENROL along with a pseudonym generated by at Data provider local level.
The medical information included in ENROL is the information gathered in the routine medical care, i.e. date of birth, gender, diagnosis including genetics, blood results, symptoms and treatments received. This information corresponds to the ‘Common Data Elements’ defined by the EU-RD-Platform for rare disease registries, as well as a set of ‘specific Elements’ related to rare haematological diseases agreed upon by the Steering Committee of ENROL.
This will mean that at the European level, ENROL will allow mapping of the following important information concerning patients affected by rare haematological diseases: demographics survival rate, methods for diagnosis, the main clinical features and treatments.
Answer
Research is often carried out in collaboration with other international researchers from public or private sectors. By sharing research data, more questions can be answered.
Pseudonymised data held by ENROL could be shared with third parties (i.e. researchers, patient organisations, health authorities, pharmaceutical and other commercial companies). These research projects will have objectives which are directly connected to ENROL’s aims. These research projects may include those seeking to increase knowledge of the disease and the development of new treatments within academic/hospital/pharmaceutical research projects. We ask specifically for patients' permission to re-use pseudonymised data for these kind of research projects.
Third parties interested in accessing data held by ENROL will be required to submit an application form that details the scientific purposes of the project for which the data is needed. Requests are reviewed by a Data Access Committee comprised of both health professionals and patient representatives that ensure that the request aligns with the purpose of ENROL. The research applicant’s professional background is also reviewed to ensure they are suitably qualified and have a track record of integrity as a researcher in this area. Researchers may come from both public and private institutions in any country, including non-EU countries. Where required by the local applicable law the research will need the approval of an Ethics Committee.
All third parties will be required to sign legal agreements requiring them to respect EU legislation and commit to: using the data only for the purpose intended and authorised; not attempting re-identification of the data, including the merging of ENROL’s data with other sources of data; and therefore, and not contacting patients directly.
You are able to find more detailed information on the legal framework and the process required to request data to ENROL at the website.
Answer
Information about projects which have been given access to ENROL data is publicly available on the ENROL website. In addition, patients can request information on the specific projects that have accessed their data directly requesting it to their medical doctor or Data Protection Officer of the Data provider.
Answer
Data will be stored in ENROL until such time as the objectives of ENROL have been met, which may be several decades from now. However, the need to keep data in ENROL will be reviewed every 15 years.
By contacting the medical doctor or the Data Protection Officer of the data provider, the patients can request access, rectification or erasure of their data in ENROL, as well as withdraw their consent at any time and without the need of justification.
Patients can ask their medical doctor the access to information on the specific projects that have accessed their data. Data providers are responsible for communicating to ENROL the requests from the data subjects.
Nevertheless, to guarantee the validity of research, data already collected and analysed cannot be deleted even if the data subject decided to withdraw from the project. If the data subject is not satisfied, they can also lodge a complaint regarding the processing of their data with the competent supervisory authority of each Country/City.
Answer
Keep updated on the breaking news in the field of Rare Hematological Diseases through the European Reference Network on Rare hematological diseases (ERN-EuroBloodNet) website and subscribe to the newsletter!
Also you can follow ERN-EuroBloodNet in Twitter, Facebook, LinkedIn and Youtube Channel!
Answer
Please contact
Answer
Only registered data providers can register new patients. Please check Q: Creating a user account with all necessary credentials for the correct procedure on how you can create a user account and be able to register patient data.
To register a new patient you need to:
- Log in to ENROL platform using your account credentials
- Click the button in List of Patients table. Alternative, you can click the main menu item found at the top of the page.
- Fill all the required data and click the button
-
Upon successful patient registration a message with the patient pseudonym will appear.
Please save the pseudonym in a secure manner for easier patient data records retrieval.
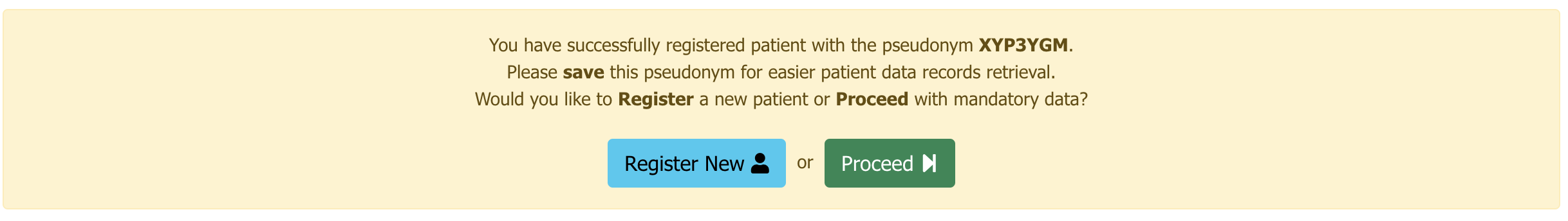
- You can choose to proceed to fill the patient Baseline data parameters by clicking the button or Register a new patient by clicking the button .
- If you choose to proceed to fill the patient Baseline data parameters, you will be redirected to the patient baseline medical form to fill all the mandatory and optional data parameters divided in different Categories and sub-sections.
- If you choose to register a new patient instead of proceeding to fill the Baseline data, you will be redirected back to the Register New patient form to fill the new patient required data. You can fill the Baseline data of each new patient by selecting the patients' pseudonym from the List of Patients table found in ENROL homepage as described in Q: Fill patient Baseline data.
Answer
Here we describe the procedure of filling a patient Baseline medical record in case you did not proceed to fill them upon successful patient registration.
To fill the patient Baseline Medical record you need to:
- Log in to ENROL platform using your account credentials
- Select the patient pseudonym you wish to fill the Baseline Medical record from the List of Patients table and click the button in column View.
- You will be redirected to patients' main medical record page where you need to click the button in column View of the Patient Medical History table.
-
You can fill all the mandatory
and optional data parameters of each Category by navigating
through the navigation bar located below the patient pseudonym.

-
Click the button
located at the bottom right of each section to save the filled data before proceeding to the next section.
For example:
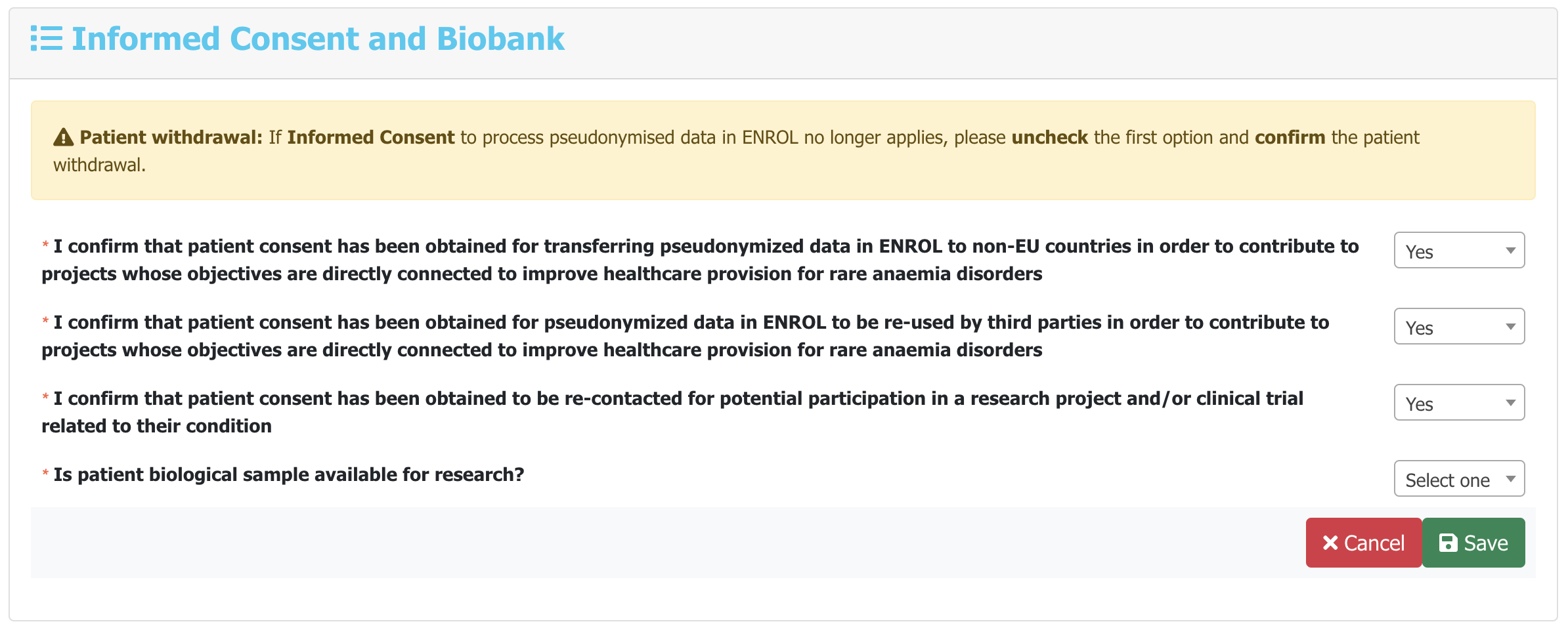
Answer
To create a patients' new follow-up you need to:
- Log in to ENROL platform using your account credentials
-
Select the patient pseudonym you want to create a new follow-up by clicking the folder icon
in List of Patients table.
-
In patients' main medical record page you can create a new follow-up by clicking the button
located at the right of the patient pseudonym.

-
You will be redirected to the New Follow-up form page where you can fill the new follow-up
data parameters. The data parameters that need to be filled in each new follow-up are emphasized in
red and bold letters for easier and faster navigation through the individual categories.
For example: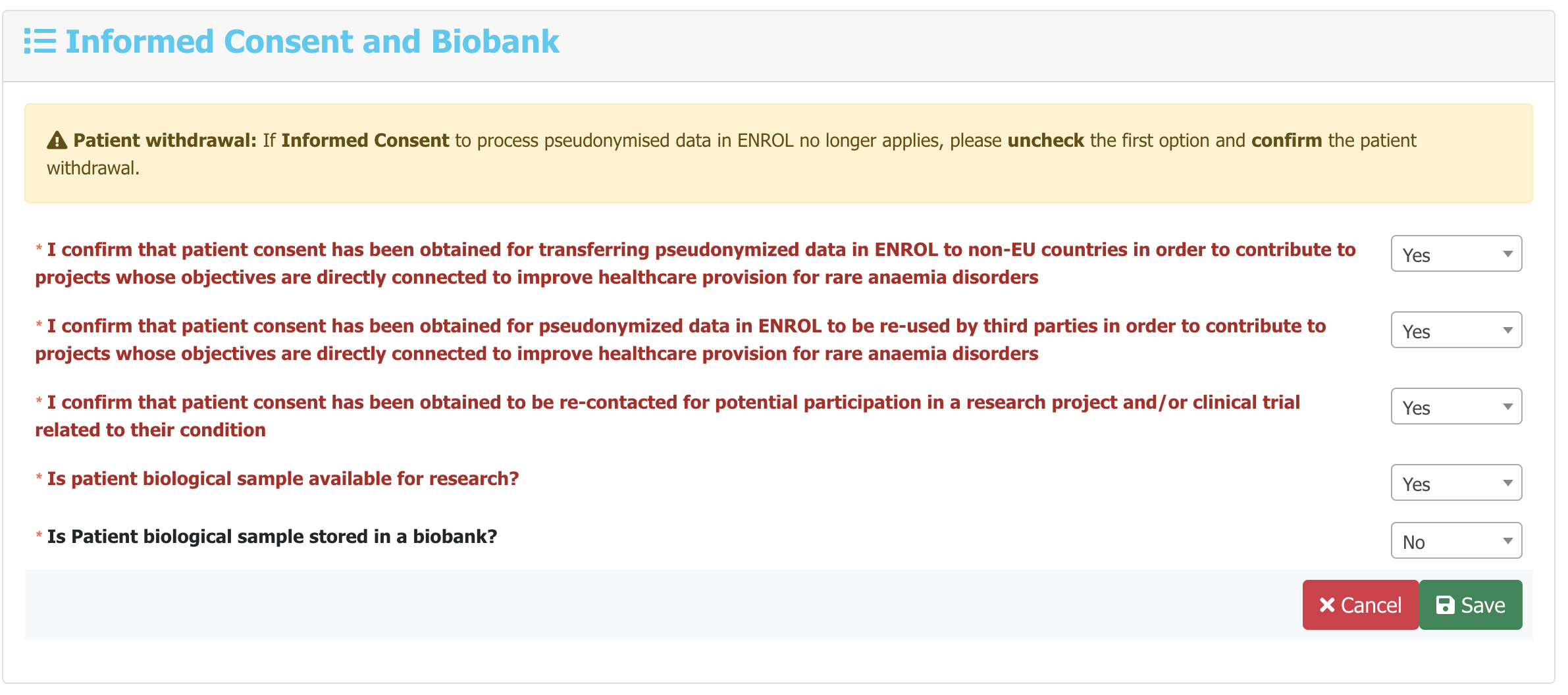
- Click the button located at the bottom right of each section to save the filled data before proceeding to the next section.
-
Confirm the new follow-up by clicking the button
or cancel all changes by clicking the button
located at the right of the patient pseudonym.

- You will be redirected back to patients' main medical record page where you can add a new follow-up or view/edit other follow-ups.
Answer
To directly edit a patients' data you need to:
- Log in to ENROL platform using your account credentials
-
Select the patient pseudonym you want to edit data by clicking the folder icon
in List of Patients table.

-
In patients' main medical record page you can access and edit a specific data record (i.e. Baseline or Follow-up)
by clicking the folder icon in column View of the Patient Medical History table.
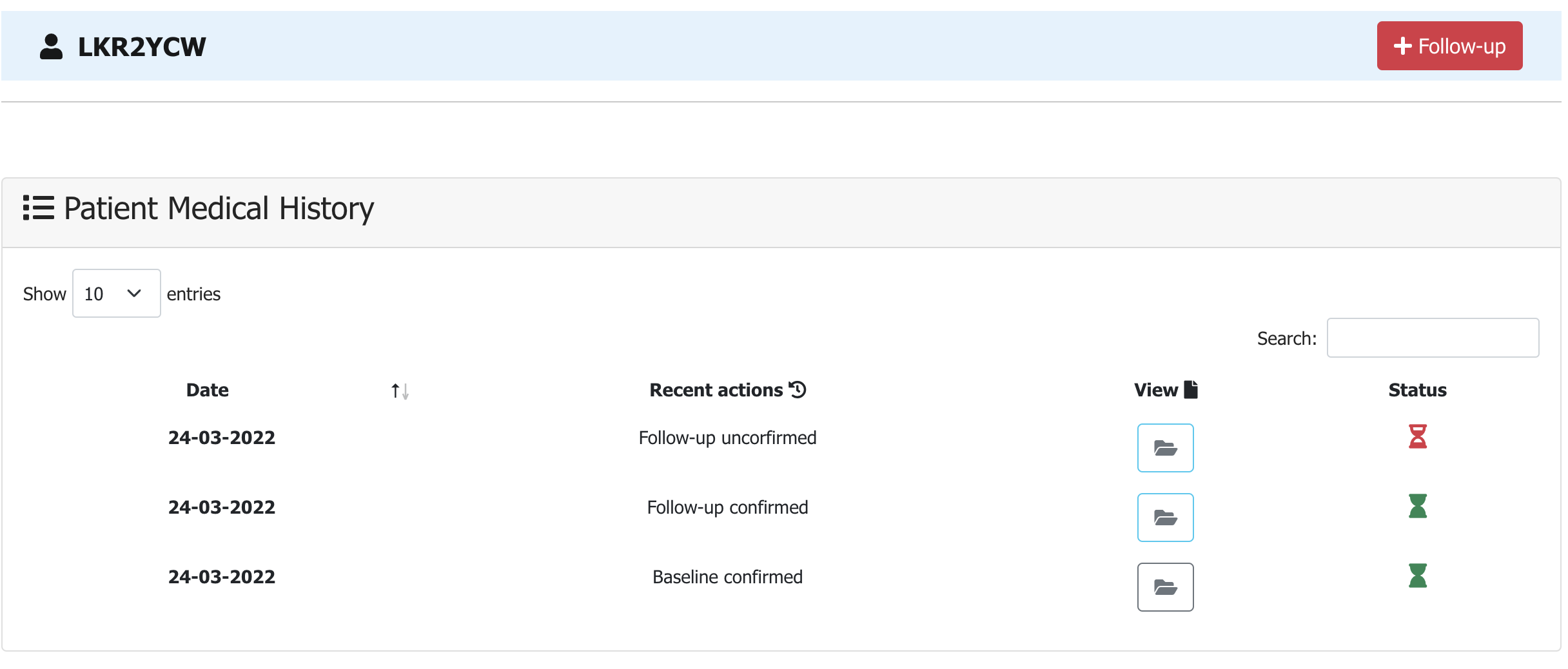
-
The selected medical record opens in editable form and you can edit all or a specific Category
by navigating through the navigation bar located below patients' pseudonym.

- Save any changes in the individual sections of each Category by clicking the button or cancel any changes by clicking the button found at the bottom right of each section.
-
Once finish all edits in the individual Categories
please Confirm all changes by clicking the button
located at the right of patient pseudonym. This will ensure that all changes were saved
and there were no errors during the editing of patient data.

- You will be redirected back to patients' main medical record page where you can add a new follow-up or view/edit other follow-ups.
