European Rare Blood Disorders Platform
Objectives
The European Rare Blood Disorders Platform (ENROL) is conceived in the core of the European Reference Network on Rare Hematological Diseases (ERN-EuroBloodNet), as the umbrella platform for both new and already existing registries on Rare Hematological Diseases (RHDs) avoiding fragmentation of data by promoting the interoperability standards for patient registries.
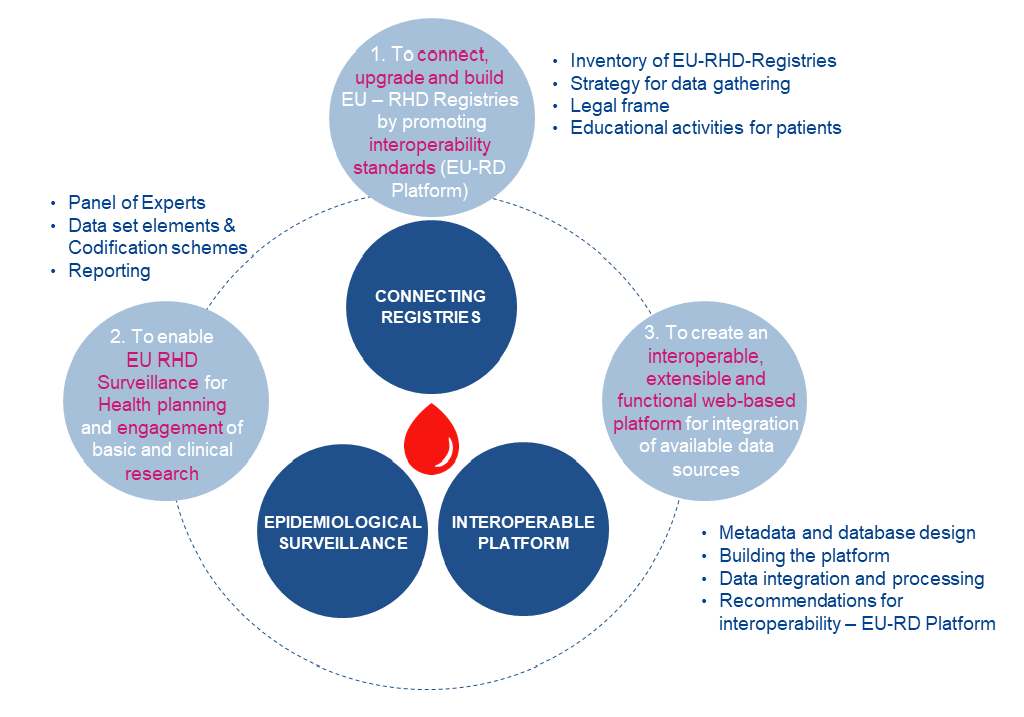
ENROL aims at mapping demographics, diagnosis methods, genetic data, main clinical manifestations and treatments at the EU level by enabling the connection, upgrading and building EU patients registries in line with EU data protection and interoperability standards with the following major objectives:
- Promoting research: allowing the identification of patient cohorts to facilitate the development of collaborative projects on basic and clinical research on RHD
- Epidemiological surveillance and health planning: pooling of available data on registries and databases across European countries to monitor trends and provide essential epidemiologic information on RHD while enabling the generation of evidence for the optimum allocation of resources and definition of health policies.
ENROL Specific Objectives
Disease Coverage and Domain Specific Pilots
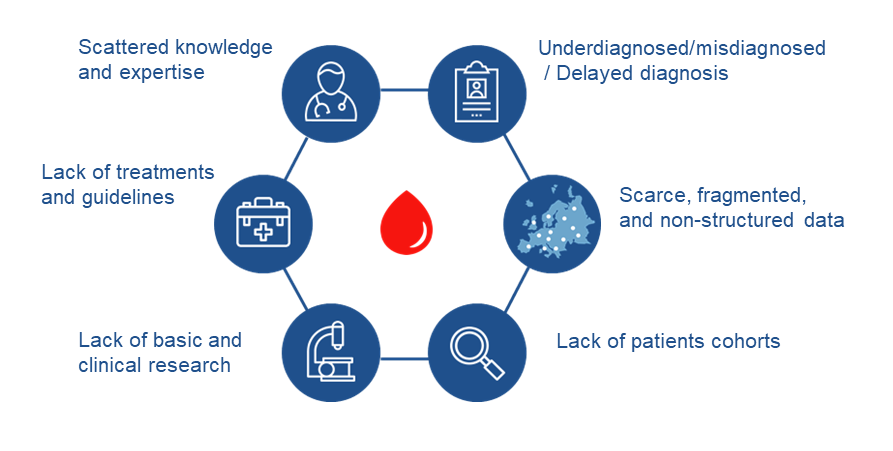
ENROL disease coverage results in more than 450 different entities with differential clinical and etiological features i.e. oncological vs non-oncological, hereditary vs acquired, or significant difference frequency, among others, which can be classified into the following disease groups:
- RAD: Inherited Rare Anaemia Disorders, including inherited Bone Marrow Failures
- BMF: Acquired Bone Marrow Failures
- Bleeding: Rare bleeding-coagulation disorders and related diseases
- HH-Iron: Hemochromatosis and other rare genetic disorders of iron metabolism and heme synthesis
- Myeloid: Myeloid malignancies
- Lymphoid: Lymphoid malignancies
ENROL dataset has been conceived in a bottom-up design for ensuring the capture of the common elements for rare diseases in line with the EU recommendations and with the EU RD platform, and the key features common for the whole spectrum for RHDs. The latest level can be stepped up for the definition of domain specific elements that support the in-depth analysis.
In the spirit of ENROL aims to promote the connection and linkage of available data sources and the creation of new registries where lacking, ENROL has established collaborations for supporting the upgrade/creation of two pilot European registries for the domain specific data gathering in compliance with the standards of interoperability and ENROL policy focussed on: Blastic plasmacytoid dendritic cell neoplasm (BPDCN) and Rare Anaemia Disorders (RADs).
European Commission strategy for RD registration
European Platform on Rare Disease Registration (EU RD Platform)
Copes with the fragmentation of RD patients data contained in hundreds of registries across Europe by releasing standards for interoperability.
European Reference Networks registries
Grants for supporting RDs registries for ERNs to:
- Enable building
- Upgrading
- Linking and making interoperable registries covering the diseases of each ERN following the standards defined by the EU RD Platform
ERNs Working group on Research includes a task force on registries with representatives of ERNs to align forces on transversal actions in collaboration with JRC and EJP-RD, as:
- ERN registry data dictionary
- Domain specific Common Data Elements
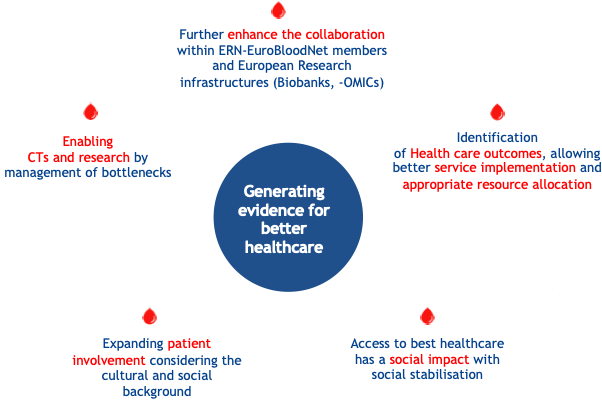
Expected Outcomes

Consortium
 Coordinator
Coordinator
Maria del Mar Mañú Pereira Vall d’Hebron University Hospital (HUVH) Vall d’Hebrón Research Institute (VHIR) Barcelona Spain |

|
 Co-Principal investigator
Co-Principal investigator
Petros Kountouris The Cyprus Foundation for muscular dystrophy research (CING) Nicosia Cyprus |


Co-Principal investigator
Béatrice Gulbis Erasme University Hospital (ERASME) LHUB-ULB Brussels Belgium |

|
 Co-Principal investigator
Co-Principal investigator
Pierre Fenaux Assistance Publique - Hopitaux de Paris (AP-HP) Hopital St Louis Paris France |
|---|
Disclaimer
ENROL is co-funded by the Health Programme of the European Union under the call for proposals HP-PJ-2019 on Rare disease registries for the European Reference Networks. GA number 947670.